How do you write a structural formula for the compound 4-ethyl-trans-2-heptene?
1 Answer
Aug 19, 2016
as follows
Explanation:
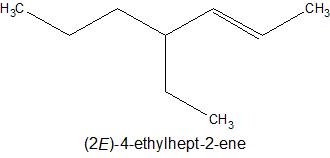
1) Draw a C-chain of length 7 as per stem name- hept.
2) Put double bond at position 2 as per suffix name- 2-ene.
3) Put an ethyl group at position 4 as per prefix-4-ethyl.
4) The ethyl group should be drawn at trans position w .r to methyl group to make it a trans isomer.
5) Put Hydrogen atoms with all C- atoms as required to satisfy its tetra valency.

