And if there are 9 such electrons, the #O# atom must bear a formal negative charge because an isolated oxygen, #Z=8#, has 8 protons, 8 positively charged particles in its nucleus. The balance, the difference, between charged fundamental particles is necessarily the charge on the ion.
So if we start with water, #H-O-H#, we know that water is a neutral molecule, and the electron dot diagram reflects this. From our earliest lessons as chemists, we know that atoms, nuclei, share electrons in a covalent bond. In an #O-H# bond, #1# electron is "owned by" hydrogen, and the other electron in the bond devolves to oxygen. The hydrogen nuclei are thus formally neutral, because their positive charge (in the nucleus) is balanced by their bonding electron.
Now to oxygen; #O# claims 1 electron each from its 2 bonds; the 4 electrons in the 2 lone pairs devolve solely to oxygen, and there are 2 inner core electrons, which are not presumed to be involved in any bonding. Around #O#, there are thus #2+2+2xx2=8" electrons"#, whose charge is precisely balanced by the nuclear protons (how many?).
We write the protonolysis reaction in the following way:
#H_2O rarr HO^(-) + H^+# (well this is one way to write this!).
Any chemical reaction preserves mass and charge, and it is certainly preserved here. And thus a negative charge is associated with the oxygen atom of hydroxide.
From here:
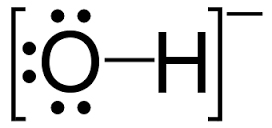
The diagram is not ideal because it does not explicitly suggest that the negative charge is localized on oxygen; c'est la vie.


