How does a cosolvent ( mixture of water and alcohol) increase the solubility sparingly Sildenafil Citrate, a drug? It is really challengable.
I have read about the dielectric constants of water and ethanol play an important role in the solubility of SC. I want your answer with explanation.
I have read about the dielectric constants of water and ethanol play an important role in the solubility of SC. I want your answer with explanation.
1 Answer
You usually do not know....solubility is an experimental proposition....
Explanation:
When you recrystallize something you juggle TWO competing criteria: (i) intrinsic solubility; and (ii) ease of crystallization of the solute. Very powerful organic solvents such as methylene chloride, or chloroform, CAN get solutes up into solution....but they never come out (i.e. the solute is too soluble in this medium). Sometimes a solution of the solute in chloroform or methylene chloride is LAYERED with a solvent such as pentane, and as the solvents SLOWLY mix (in a fridge say), crystals of solute can appear at the interface...
The solute you got here,
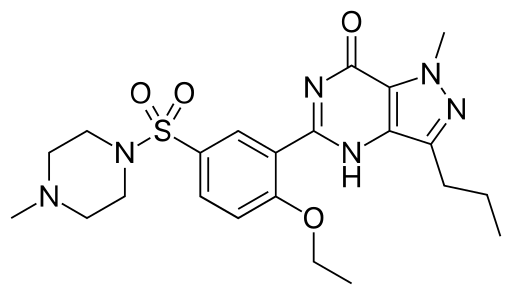
...is a large organic molecule with lots of polar groups....possibly would go up into methylene chloride...a cosolvent WOULD have to be added to get it out...but again what works, works...this is entirely the province of experiment.

