How does electromagnetic radiation relate to the structure of an atom?
1 Answer
You can consider, for example, the hydrogen atom (the simpler with one electron only) and its structure.
In a classical "planetary" model, the electron would orbit the nucleus like a planet orbits the Sun but this doesn't work because classically the electron (a charged particle) going around (with centripetal acceleration) would emit radiation (energy) and rapidly fall into the nucleus (radiation is emitted by accelerated charges, have a look at the idea behind an antenna).
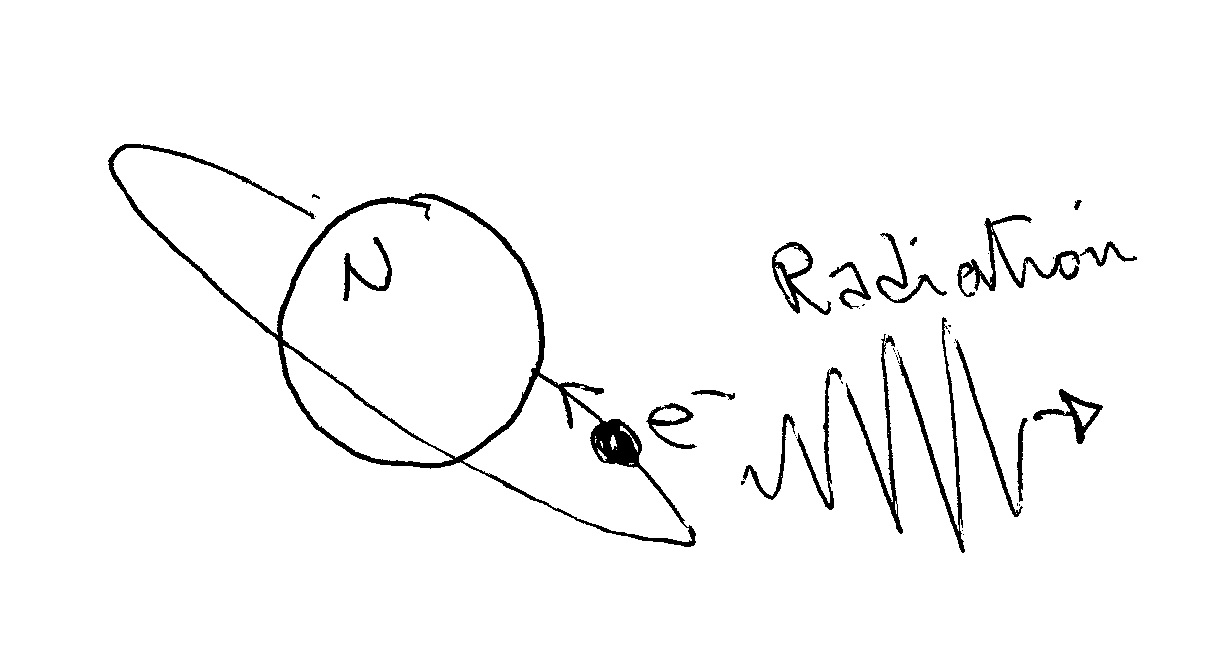
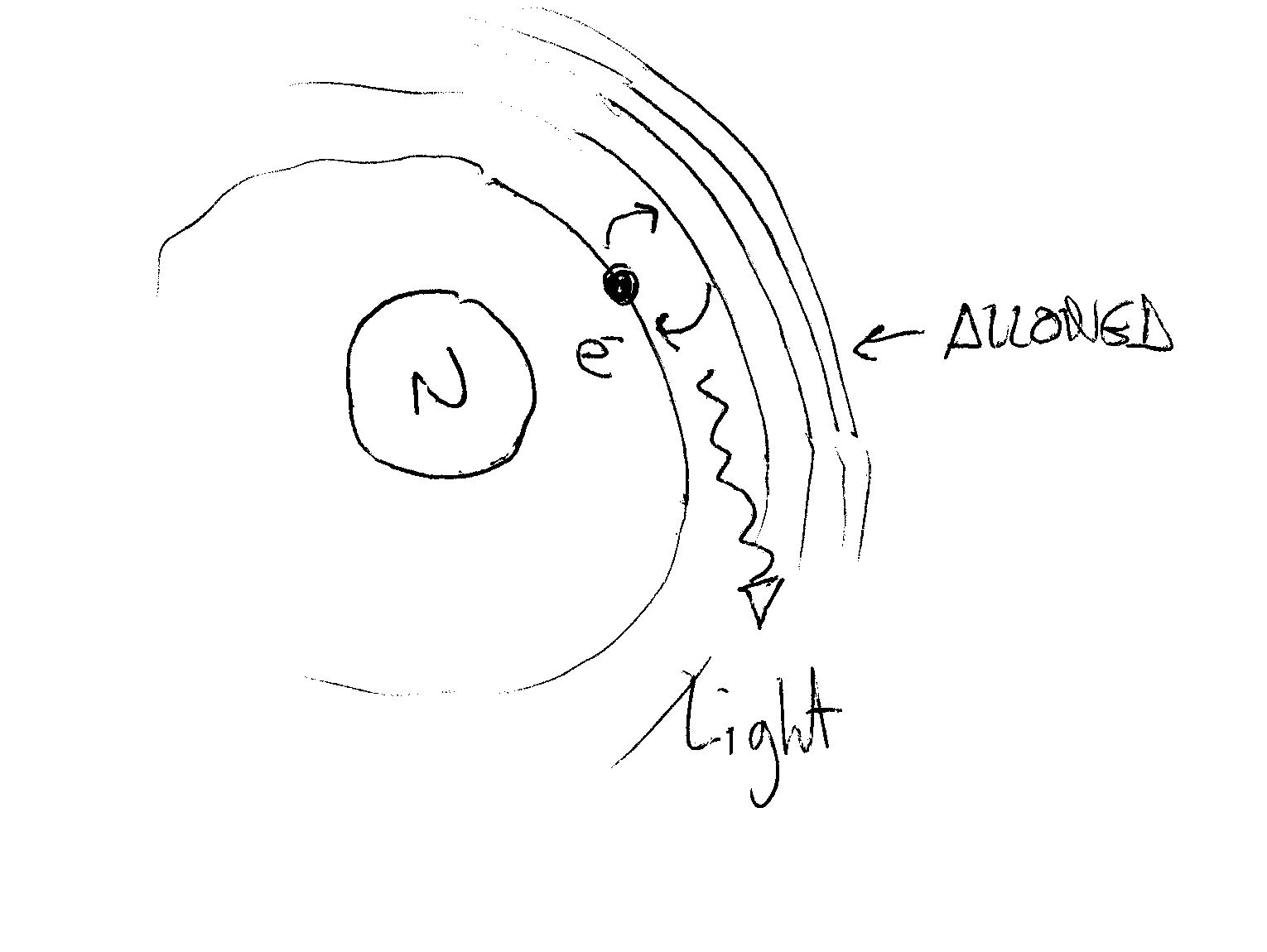
In the quantum mechanical model devised by Niels Bohr you have a nucleus and an electron orbiting on "allowed" orbits. On these orbits (placed at fixed, quantized, intervals) the electron can orbit WITHOUT emitting radiation (and so without loosing energy).
Radiation comes into our picture when the electron changes orbit!!!!
When you give energy to the atom the electron jumps, say, from the first to the second orbit and then rapidly goes back to the original orbit EMITTING a quanta of light (of energy equal to
When you give energy to a sample of hydrogen (using current, heating it or illuminating it) electrons can jump to various orbits and going back emit the energy they received at various frequencies (corresponding to the "height" of the various jumps) in form of electromagnetic radiation, some of which can be in the visible (have a look to the Balmer Series for Hydrogen):
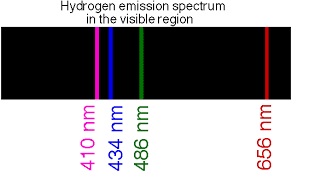
Hope it helps

