How does enthalpy change with temperature?
1 Answer
Enthalpy will change depending on the temperature.
Explanation:
When we calculate enthalpy, the sum of internal energy and the product of pressure and volume, we calculate it for a specific temperature.
If you were to increase the temperature, you would also increase the energy of the molecules, meaning those molecules interact with each other at a greater rate. Thus, you have increased your internal energy. Therefore, you would also expect your enthalpy to increase (because enthalpy is internal energy+(pressure*volume)).
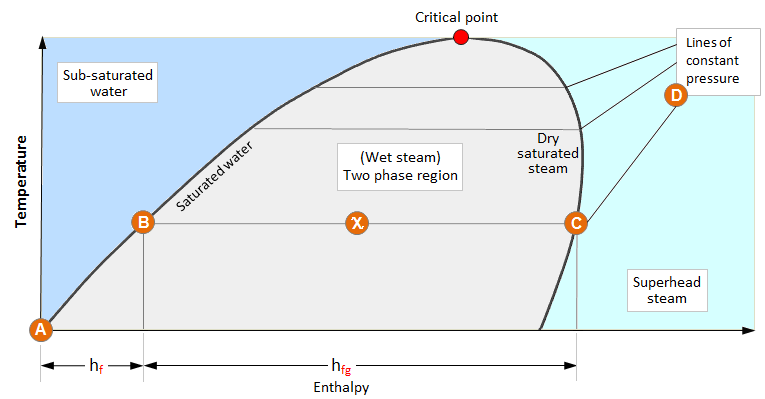
If you were to keep increasing the temperature, the substance might eventually undergo a phase change (for example, heat up enough water and it becomes steam). At the critical point, where the phase change occurs, you would see a decrease in temperature and enthalpy of the substance, as shown below.