How does ionization energy change with the size of atoms?
1 Answer
Would not the larger atom have the INTRINSICALLY SMALLER ionization energy?
Explanation:
The ionization energy is defined by the process......
But we should look at the data.......
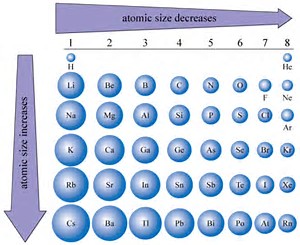
Clearly, atomic size DECREASES across the Period, from left to right as we face the Table.
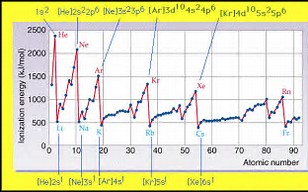
And also clearly, ionization energy INCREASES across the Period from left to right as we face the Table. And this trend ALSO makes sense on the basis of smaller atomic radii, as well as on the basis of greater atomic charge. Because electrostatic attraction varies inversely to the square of electronic radius, smaller atoms should have intrinsically greater ionization energies. Do these data support these arguments?

