How does zinc form an insoluble zinc hydroxide in water?
1 Answer
It does so in moderately basic solution, modeled by the reaction seen here. Note that in overly basic solution,
Consider the following Pourbaix Diagram:
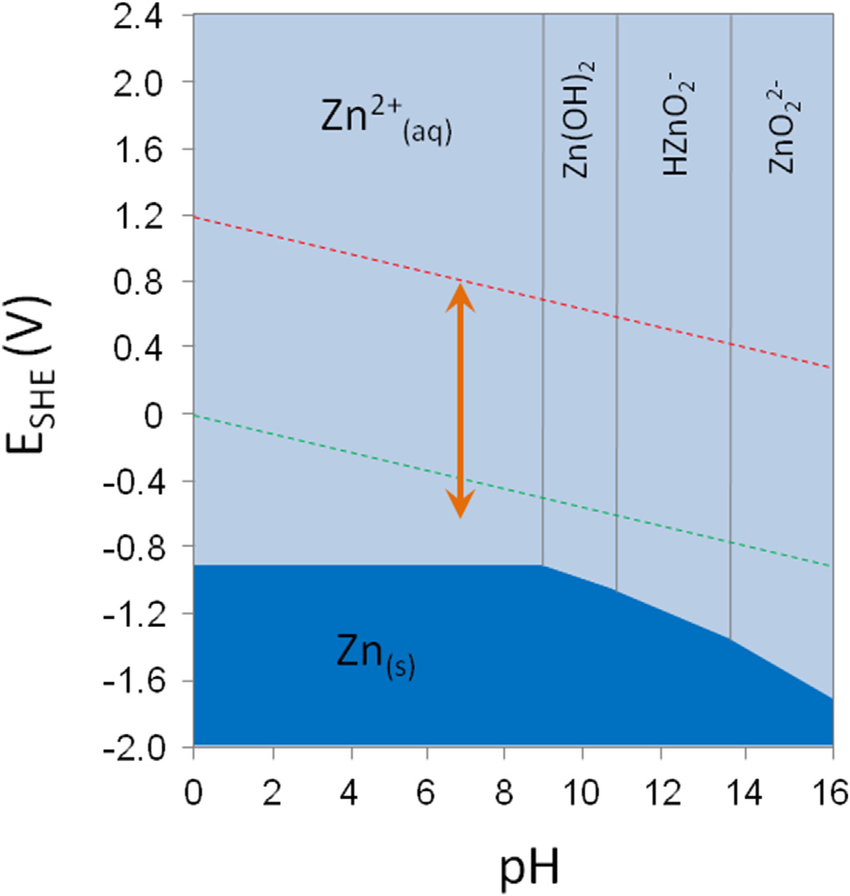
This graphs the EMF of the redox process against the
The higher up you go in the diagram, the more oxidized the species are, and the farther right you go, the more base-ic the species become.
You can see
#"Zn"(s) -> "Zn"^(2+)(aq) + cancel(2e^(-))#
#2"H"^(+)(aq) + cancel(2e^(-)) -> "H"_2(g)#
#"--------------------------------------------------"#
#"Zn"(s) + 2"H"^(+)(aq) -> "Zn"^(2+)(aq) + "H"_2(g)#
In basic conditions, we just have to add
#"Zn"(s) + 2"H"^(+)(aq) + 2"OH"^(-)(aq) -> "Zn"^(2+)(aq) + 2"OH"^(-)(aq) + "H"_2(g)#
This gives:
#color(blue)("Zn"(s) + 2"H"_2"O"(l) -> "Zn"("OH")_2(s) + "H"_2(g))#
which is the reaction you asked about here.

