How is the equivalent weight of As2S3 related to its molecular weight in the following reaction : As2S3 + H+ + NO3- ------> NO + H2O + AsO4 3- + SO4 2- ?'
1 Answer
Answer is 123.01g/equivalent
Explanation:
This is a redox reaction , so in order to determine the equivalent weight in this type or reactions, we have to follow this equations:
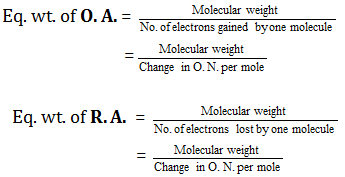
where; O.A is an oxidizing agent and R.A is a reducing agent.
First, we can obtain the molecular weight of Arsenic sulfide from the Periodic Table of elements which is 246.02g/mol .
Second, we have to follow the change in oxidation number of As before the reaction and after it to determine the valency.
-As a reactant , the oxidation number of As in As2S3 is (+3)
-As a product , the oxidation number of As in (AsO4)-3 is (+5)
Hence, the change in oxidation number is 2 which is the absolute difference between them.
By substitution in the equation, the equivalent weight of As2S3 is 123.01g/equivalent .

