How is the periodic table arranged?
1 Answer
Feb 13, 2014
The elements in the Periodic Table are arranged according to increasing atomic number.
As you go horizontally from left to right across a Period in the Periodic Table, you are adding one more proton to the nucleus (increasing the atomic number by one).
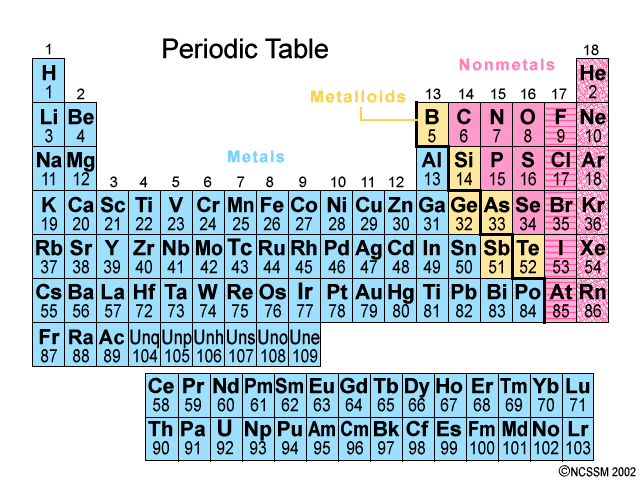
Elements with similar properties are arranged one above the other in vertical Groups numbered from 1 to 18.
Metals (blue) are on the left; nonmetals (pink) are on the right; metalloids (yellow) lie along the zigzag line that divides the metals and nonmetals. The noble gases are on the far right.

