How is the speed of a chemical reaction related to the spontaneity of the reaction?
1 Answer
It's not. Spontaneity is a thermodynamic phenomenon, and the rate/speed of a reaction is a kinetic phenomenon.
The rate of a chemical reaction can be written in a rate law as
#r(t) = k[A]# where
#r(t)# is the rate of reaction.#k# is the rate constant for that reaction at a given temperature.#[A]# is the concentration of#A# in#"M"# .
for a simple unimolecular reaction
#A -> B# ,
which has activation energy
Both are on the same reaction coordinate diagram, but they do not influence each other.
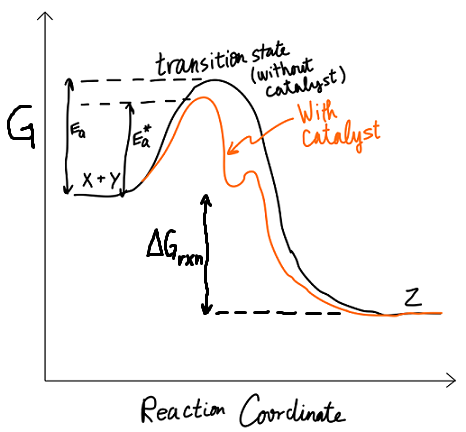
Modifying the rate
From the Arrhenius equation
#k = Ae^(-E_a"/RT")# ,
increasing
Yes,
When you modify
- If the overall reaction was spontaneous without a catalyst, it is still spontaneous with a catalyst.
- If it is also slow without a catalyst, it is now fast with a catalyst, but not additionally and suddenly possible/spontaneous.

