How is thermochemistry used for phase changes?
1 Answer
Jun 5, 2018
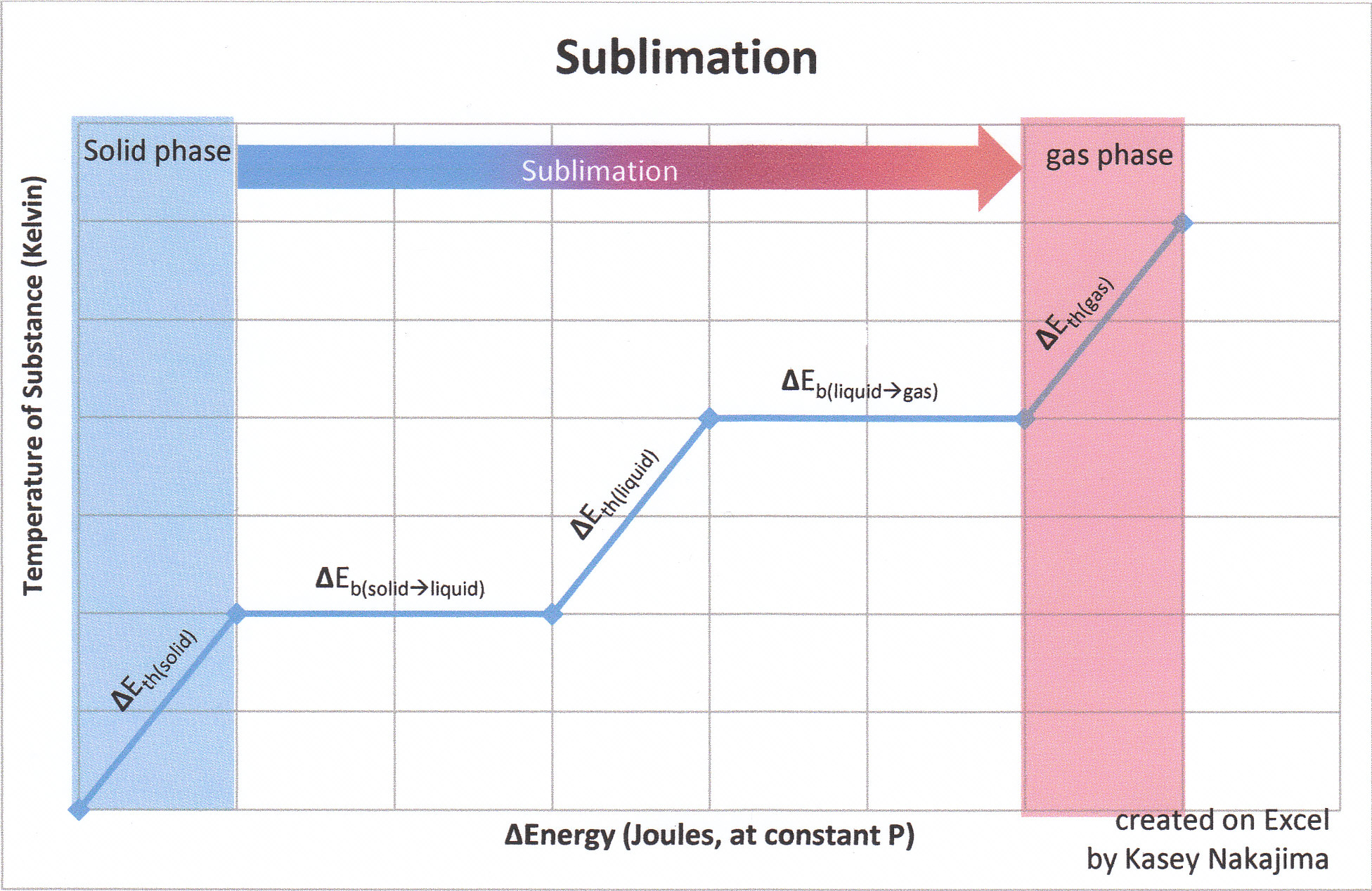
Thermochemistry is used to calculate the amount of energy required to change from state to state.
Explanation:
For example, if you need to change from solid water to liquid, there is a constant known as heat of sublimation (specific heat), which has been experimentally determined. However, there is a constant for all liquids and that can be derived with simple algebra. Without making things complicated, here is a table to help guide you: