How many molecules are in 35.5 g of #"Cl"_2# ?
1 Answer
Explanation:
Your strategy here will be to
- use the molar mass of chlorine gas,
#"Cl"_2# , to calculate how many moles you have in that sample- use Avogadro's number to convert the number of moles to number of molecules
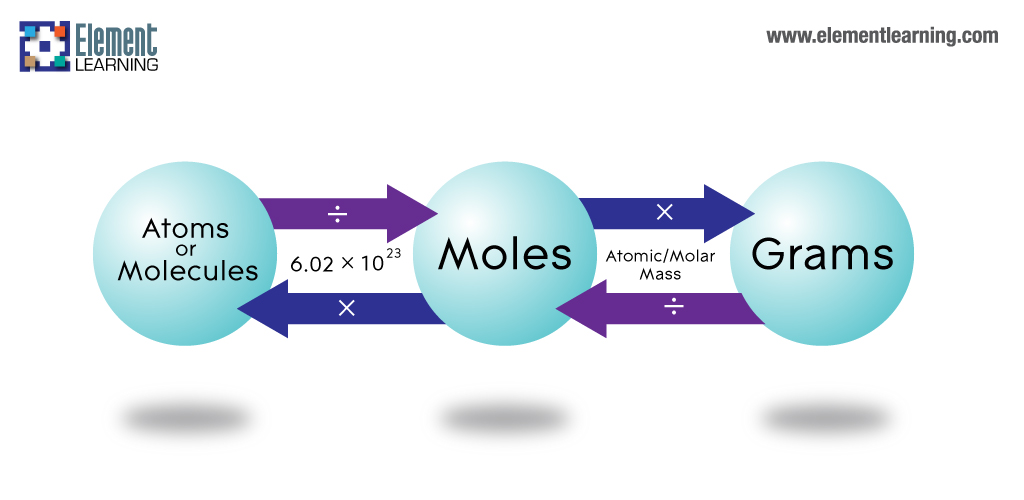
Chlorine gas has a molar mass of
#35.5 color(red)(cancel(color(black)("g"))) * "1 mole Cl"_2/(70.906color(red)(cancel(color(black)("g")))) = "0.5007 moles Cl"_2#
Now, a mole of a molecular compound is defined as
In your case, the sample of chlorine gas will contain
#0.5007 color(red)(cancel(color(black)("moles Cl"_2))) * (6.022 * 10^(23)"molec. Cl"_2)/(1color(red)(cancel(color(black)("mole Cl"_2)))) = color(green)(|bar(ul(color(white)(a/a)color(black)(3.02 * 10^(23)"molec Cl"_2)color(white)(a/a)|)))#
The answer is rounded to three sig figs.

