How many atoms are present in 48.60 g of Mg?
1 Answer
Explanation:
In order to go from mass of magnesium to atoms of magnesium, we have to do two things:
-
Convert mass of Mg to moles of Mg using the molar mass of Mg as a conversion factor
-
Convert moles of Mg to atoms of Mg using Avogadro's number (
#6.02xx10^(23)# ) as a conversion factor
Before we start, I should note that the molar mass of Mg is
We'll use this relationship:
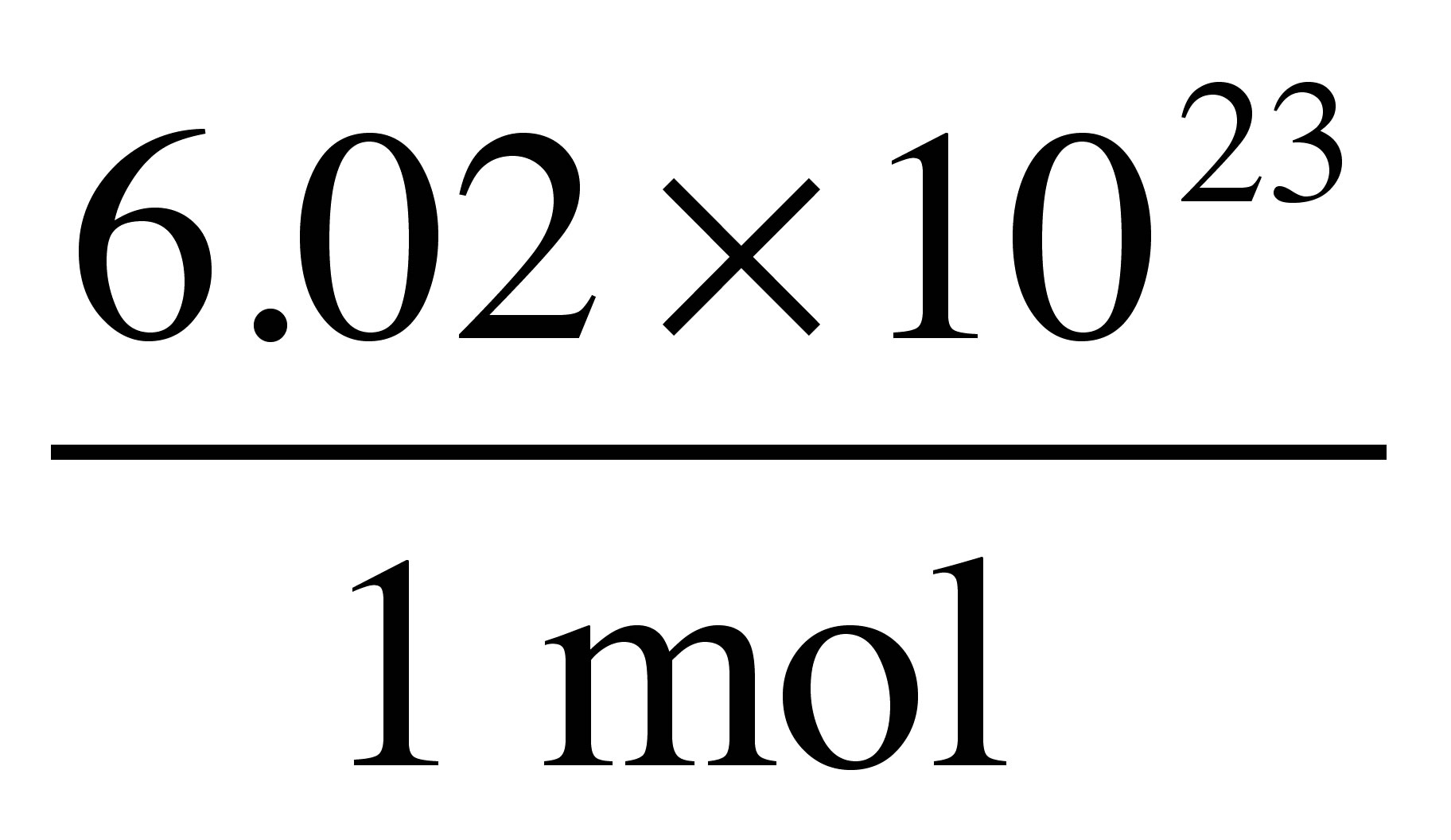
Using the moles of Mg that we just obtained, we can use Avogrado's number to perform dimensional analysis in order to cancel out units of

