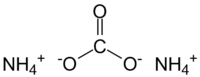
How many atoms of nitrogen #(N)# are found in #(NH_4)_2CO_3#?
1 Answer
Jan 14, 2016
Explanation:
You're dealing with ammonium carbonate,
Now, it's important to realize that in order for ammonium carbonate to be neutral, you need the overall positive charge of the cation to balance the overall negative charge of the anion.
This is what that
Since one ammonium cation contains
- one nitrogen atom,
#1 xx "N"# - four hydrogen atoms,
#4 xx "H"#
it follows that two ammonium cations will contain
- two nitrogen atoms
- eight hydrogen atoms
Therefore, one formula unit of ammonium carbonate will contain two atoms of nitrogen, one from each ammonium cation.