How many atoms or molecules are present in 1.0 mol of Au? How am I able to find the answer?
1 Answer
May 11, 2015
You use the fact that 1 mole of any substance contains exactly
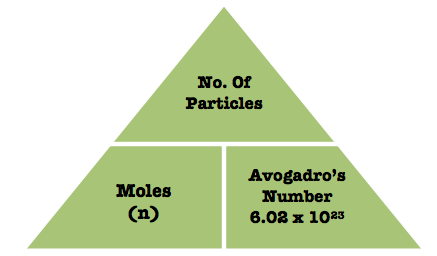
In your case, 1 mole of gold will have exactly
If round the answer to two sig figs, the number of sig figs in 1.0, you'll get
Here's a video which illustrates how to solve a problem converting from
video from: Noel Pauller
And here is an advanced example...
video from: Noel Pauller


