How many chelate rings are present in each of the following complexes?;["Cu"("trien")]^(2+), ["Fe"("ox")_3]^(3-), ["Ru"("bpy")_3]^(2+), and ["Co"("dien")_2]^(3+). Thanks
1 Answer
Apr 27, 2018
Three; three; three; four.
Explanation:
The number of rings equals the number of bonds you can cut without having the ion fall into two pieces.
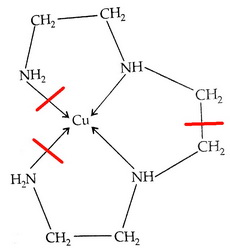 Cu(trien)2+
Cu(trien)2+
(Adapted from Sign of truth)
In the above diagram, I can make three cuts, so there are three chelate rings.
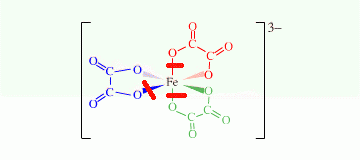 Fe(ox)3
Fe(ox)3
(Adapted from Chemical Entity Data Page)
I can make three cuts, so there are three chelate rings.
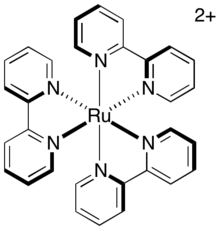 upload.wikimedia.org
upload.wikimedia.org
Now that to know the technique, can you identify the three chelate rings in this ion?
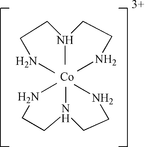 Co(dien)2
Co(dien)2
(From IUCr Journals)
Here, there are four chelate rings.

