How many electrons are in the valence shells of (a) Be in BeCl2 (b) B in BCl3 (c) H in H2O ?
1 Answer
Apr 8, 2015
(a) Be has 4 valence electrons in the compound
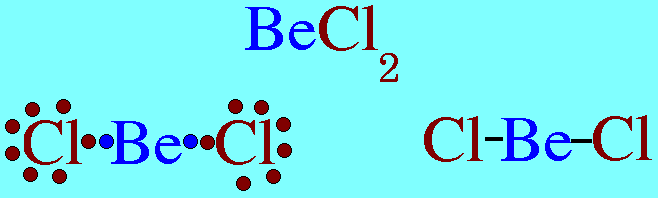
(b) B has 6 valence electrons in the compound
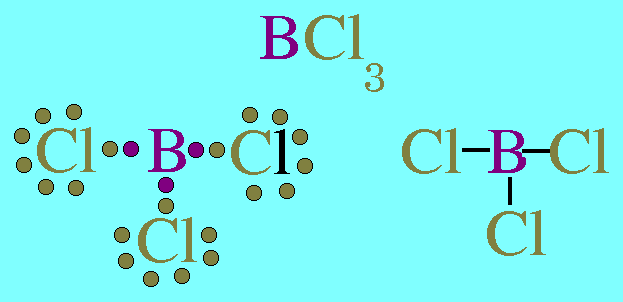
(c) Each H atom in


