How many grams of #NaOH# are required to prepare 200 mL of a 0.450 M solution?
1 Answer
3.60g of NaOH is required to prepare that solution
Explanation:
Let's begin with the equation for molarity:
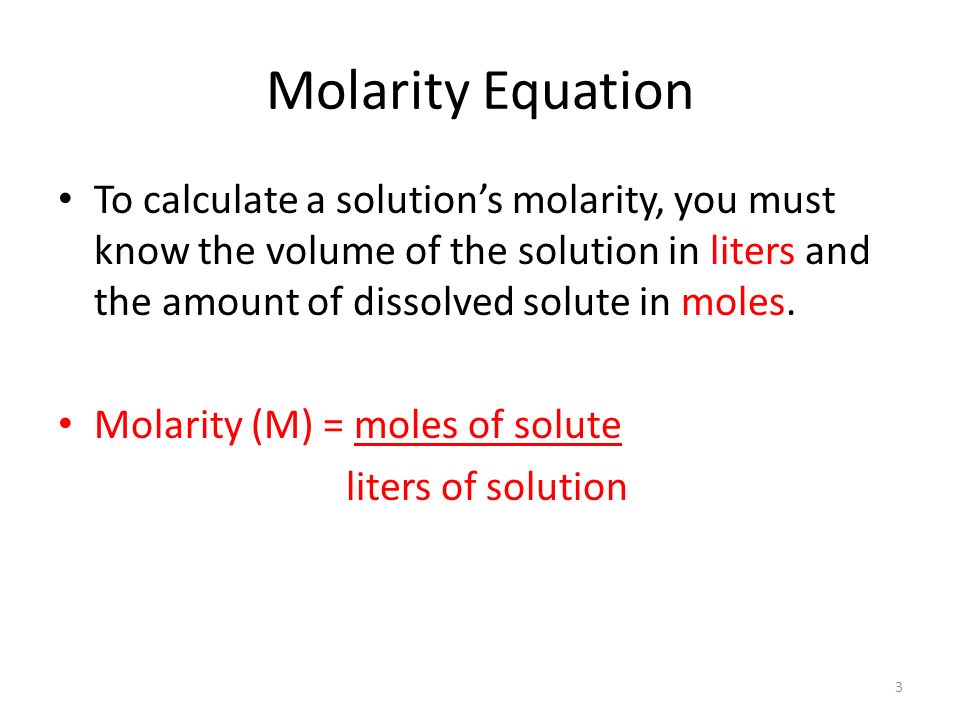
We are given the molarity and the volume of solution. The only issue is that the volume is given in mL instead of L. This issue can be fixed by using the following conversion factor:
Therefore, if we divide 200mL by 1000mL we will obtain a value of 0.200 L.
Rearrange the equation to solve for moles of solute:
Moles of solute = Molarity
Multiply 0.450 M by 0.200:
To obtain the mass of solute, we will need to the molar mass of NaOH, which is 40.00 g/mol:
Finally, multiply the number of moles by 40.00 g/mol
Boom, you have a mass of:

