How many grams of salt can be dissolved in 100 g of H2O at 80 degrees Celsius?
1 Answer
About
Explanation:
You can determine how many grams of sodium chloride, commonly known as table salt, can be dissolved in
A substance's solubility graph tells you how its solubility changes, let's say starting from room temperature, when temperature is either decreased or increased.
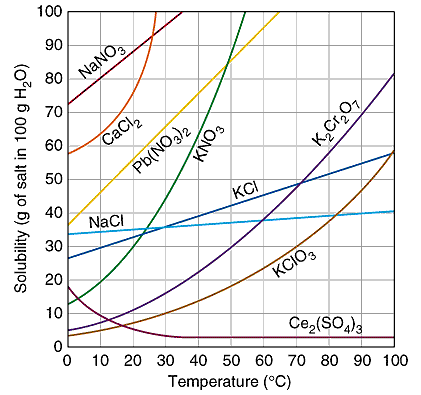
As you can see, at room temperature sodium chloride has a solubility of approximately
As temperature increases, its solubility increases as well. Notice, however, that it does not increase significantly. In fact, you can expect to be able to dissolve no more than
So, at

