How many grams of water can be produced the combination of 8 grams of oxygen and 8 grams of hydrogen?
1 Answer
This is a stoichiometry problem. You have to write a balanced equation out first
Explanation:
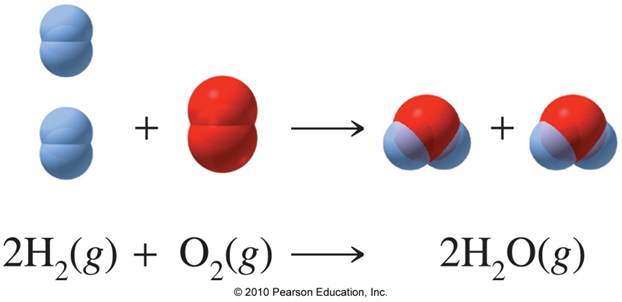
As you can see, you need 2 moles of H2 gas and 1 mole of O2 to form 2 moles of H2O.
You are given grams of both hydrogen gas and oxygen. You must find the limiting reagent first. To do this, take the mass of the reactant and convert it into moles.
Now do the mole-to-mole ratio to find out how many moles of
Ex. For
#"8 grams H"_2 * "1 mole"/(2 "g"/"mole") * ("2 moles H"_2"O")/("2 moles H"_2) = "moles of H"_2"O produced"#
For
#"8 grams O"_2 * "1 mole"/(32"g"/"mole") * ("2 moles H"_2"O")/"1 mole O"_2 = "moles of H"_2"O produced"#
Once you find the limiting reagent, proceed with finding the mass in grams of


