How many high-energy bonds does ATP contain?
1 Answer
ATP has two 'high-energy' bonds.
Explanation:
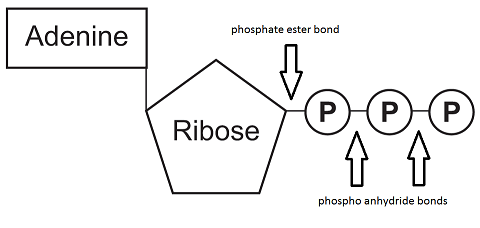
Adenosine triphosphate (ATP) consists of the nucleobase adenine, the monosaccharide ribose and three phosphate groups.
The first phosphate group is connected to the ribose with a phospate ester bond i.e. phosphate condensed with a hydroxyl (OH) group. This is a fairly stable bond and does not qualify as a high-energy bond. Adenosine monophosphate (AMP) therefore doesn't carry enough energy to power cellular processes.
The bond between two phosphate groups is a phosphoanhydride bond. This bond is less stable and is considered a high-energy bond. Adenosine diphosphate (ADP) can provide more energy than AMP.
In ATP there are three phosphate groups with two high-energy bonds as shown in the image below. The bond with the outermost phosphate group has the most potential energy and is prone to hydrolysis. ATP is therefore a very good provider of energy for cellular processes.