How many lone pairs are present on carbon?
 pc
pc
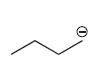
How many lone pairs of electrons (if any) are present on the carbon with negative formal charge?
pc
How many lone pairs of electrons (if any) are present on the carbon with negative formal charge?
1 Answer
There is the ONE lone pair on the carbanion....
Explanation:
There are 7 electron around the carbanion carbon.....3 electrons from the shared covalent bonds of
Because the ipso carbon claims 7 electrons,
Note that this is not a mere formalism: butyl-lithium contains the species as a polar organometallic molecule, and is widely used in organic chemistry, and represents this formalism.

