How many molecules are in 48.90 grams of water?
1 Answer
Explanation:
In order to figure out how many molecules of water are present in that
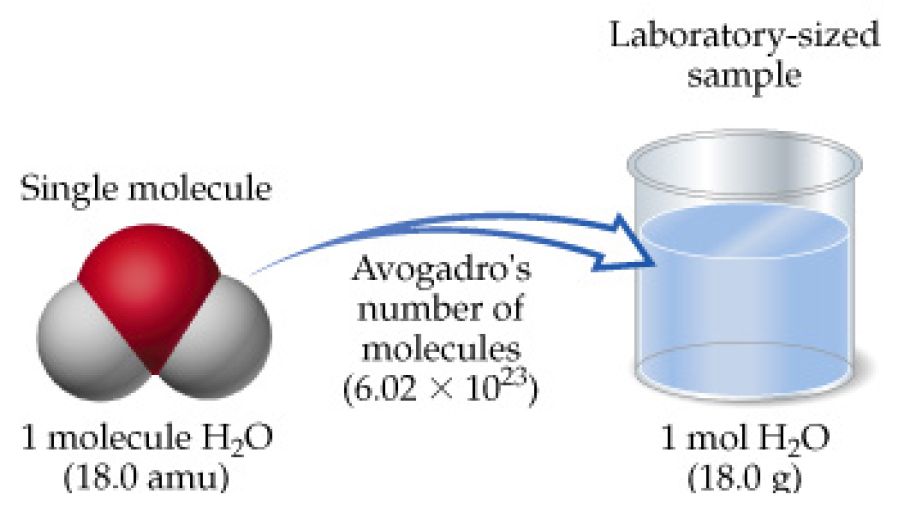
As you know, a mole is simply a very large collection of molecules. In order to have one mole of something, you need to have exactly
In order to get to moles, you must use water's molar mass. A substance's molar mass tells you the mass of one mole of molecules of said substance.
Water has a molar mass of
So, to sum this up,
 http://wps.prenhall.com/wps/media/objects/165/169519/blb9ch0304.html
http://wps.prenhall.com/wps/media/objects/165/169519/blb9ch0304.html
So, use water's molar mass to find the number of moles present in that sample
48.90 color(red)(cancel(color(black)("g"))) * ("1 mole H"_2"O")/(18.015color(red)(cancel(color(black)("g")))) = "2.7144 moles H"_2"O"
Now use Avogadro's number to find the number of molecules of water
2.7144 color(red)(cancel(color(black)("moles H"_2"O"))) * (6.022 * 10^(23)"molec.")/(1color(red)(cancel(color(black)("mole H"_2"O")))) = color(green)(1.635 * 10^(24)"molec.")
The answer is rounded to four sig figs, the number of sig figs you have for the mass of water.

