How many molecules are there in 200 grams of #C Cl_4#?
1 Answer
Explanation:
In order to be able to find how many molecules of carbon tetrachloride,
You're going to have to use two conversion factors, one to take you from mass to moles, and the other one to take you from moles to molecules.
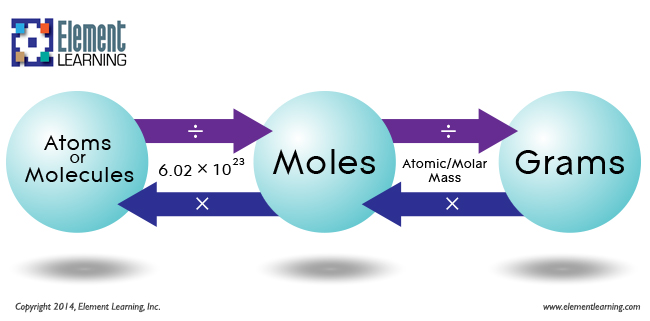
The first conversion factor is actually the compound's molar mass, which tells you the mass of one mole of a given substance.
In this case, carbon tetrachloride has a molar mass of
#200 color(red)(cancel(color(black)("g"))) * overbrace("1 mole CCl"_4/(153.8color(red)(cancel(color(black)("g")))))^(color(purple)("molar mass of CCl"_4)) = "1.30 moles CCl"_4#
Now that you know how many moles you have present in the sample, use the second conversion factor, which is Avogadro's number.
#color(blue)(|bar(ul(color(white)(a/a)"1 mole" = 6.022 * 10^(23)"molecules"color(white)(a/a)|))) -># Avogadro's number
In your case, the sample will contain
#1.30 color(red)(cancel(color(black)("moles"))) * overbrace((6.022 * 10^(23)"molecules")/(1color(red)(cancel(color(black)("mole")))))^(color(brown)("Avogadro's number")) = 7.83 * 10^(23)"molecules"#
Rounded to one sig fig, the number of sig figs you have for the mass of carbon tetrachloride, the answer will be
#"no. of molecules" = color(green)(|bar(ul(color(white)(a/a)8 * 10^(23)"molecules"color(white)(a/a)|)))#

