How many molecules of ethane are present in 64.28 liters of ethane gas at STP?
1 Answer
Explanation:
We are at STP, so we have to use the ideal gas law equation: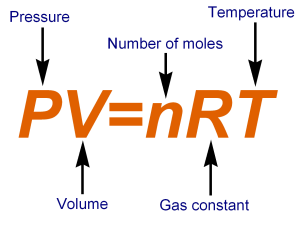
- P should have units of atm, depending on the units of the gas constant
- V must have units of liters
- n will have units of moles
- R has units of
#(Lxxatm)/ (molxxK)# - T must be in Kelvins.
Next, list your known and unknown variables. Our only unknown is the number of moles. Our known variables are P,V,R, and T.
At STP, the temperature is 273K and the pressure is 1 atm. R is equal to 0.0821
Now we have to rearrange the equation to solve for n. Finding n will allow us to use another conversion factor that will help us obtain the molecules of ethane. I'll explain that after this:
Next, we can go directly from moles of ethane to molecules of ethane using this relationship:
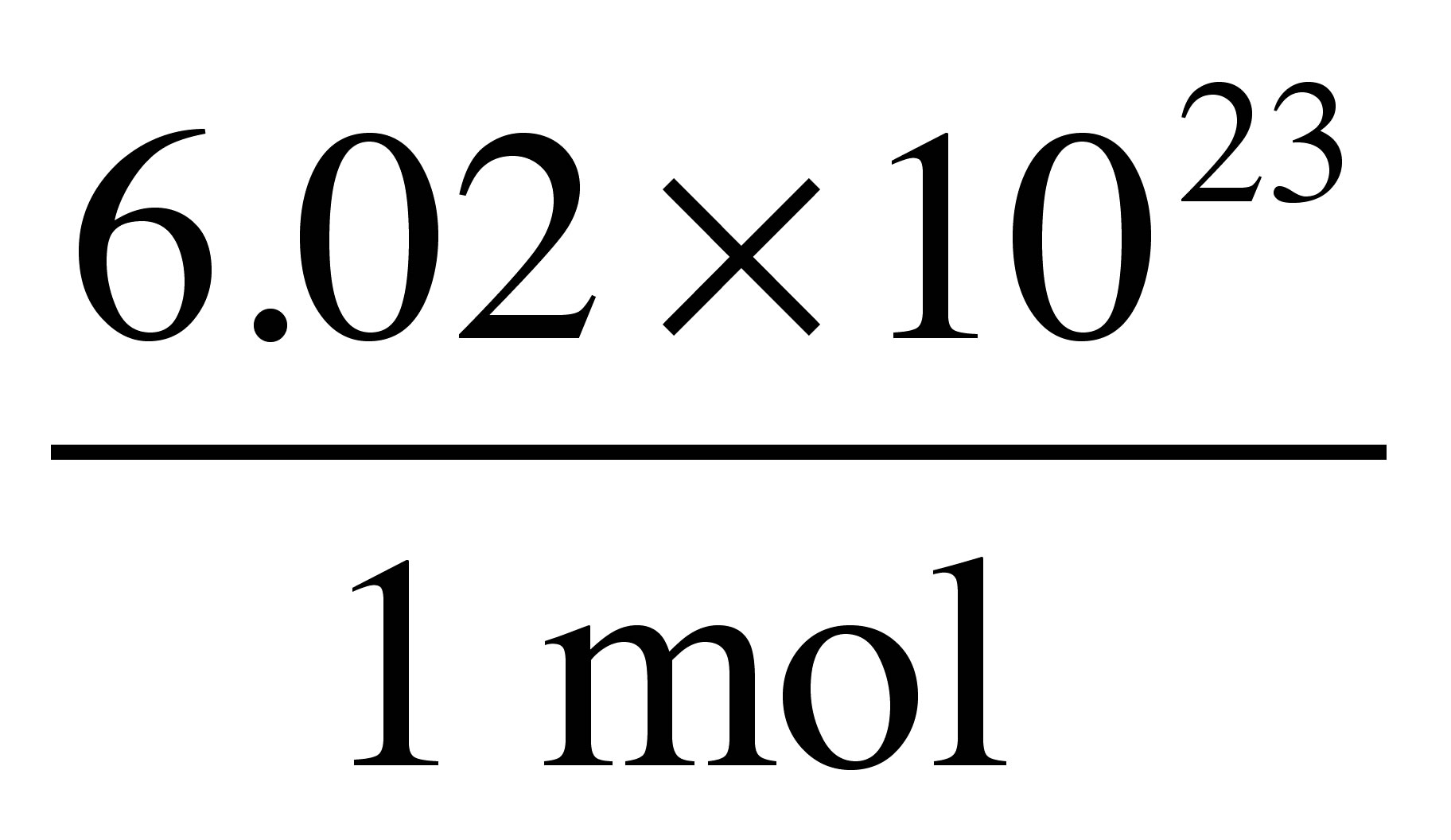
As you set up this problem, you want the units of moles to cancel out so you can end up with units of molecules:

