How many moles of He are in 16 g of the element?
1 Answer
May 1, 2017
Explanation:
Look at the periodic table and find the molar mass of Helium.
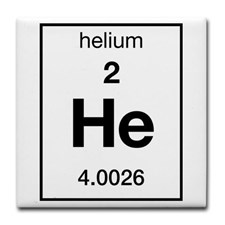
This tells us that
If we are given
Look at the periodic table and find the molar mass of Helium.

This tells us that
If we are given