How many monochlorinated products of methyl cyclohexane are optically active?
1 Answer
For cyclic ring like structure,You have to divide it in such a manner by which it looks different in its two halves.
Explanation:
Ok, see the picture. Here Imagine the methyl group is attached to the one number carbon, Then you can number the other carbon from 1-6 in the ring as per your wish.If you draw a line from one number carbon you will get two equal halves (maybe you get confuse because of methyl group but don't consider it here as it is not under any half portion). But others like 2,3,5 and 6 are chiral carbon because if you draw a line from 2 number carbon you will get two unequal halves. In one side you will get methyl and in other side there is no group. And the methyl is not chiral if it is monochlorinated because it has 2 H atom,1 cl and one -C6H5 group and we know that chiral carbon must be attached to 4 different atoms.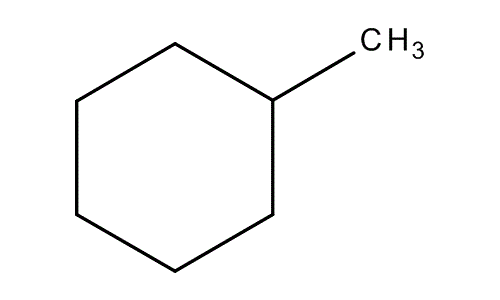 More over as like 1st number carbon 4 th number carbon also show same type of configuration .It means it is not also chiral.So there is 4 optically active chiral carbon
More over as like 1st number carbon 4 th number carbon also show same type of configuration .It means it is not also chiral.So there is 4 optically active chiral carbon

