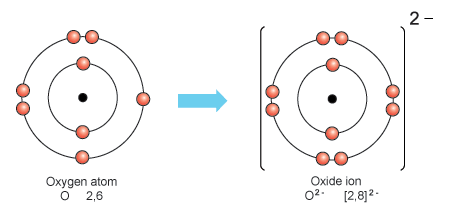
How many protons and how many electrons are in an oxygen ion that has a -2 charge?
1 Answer
Dec 30, 2016
An oxide ion has 8 protons and 10 electrons.
Explanation:
Oxygen has atomic number eight. This means that the nuclei of all oxygen atoms have eight protons. A neutral oxygen atom will also have eight electrons.
In an oxygen ion with a
This results in the oxygen ion having an octet (8) of valence electrons.