How many silver atoms are there in 4.01 g of silver?
1 Answer
Jul 12, 2016
Explanation:
Okay, so this is a two step question. By that I mean we have to go from grams of silver to moles of silver to atoms of silver:
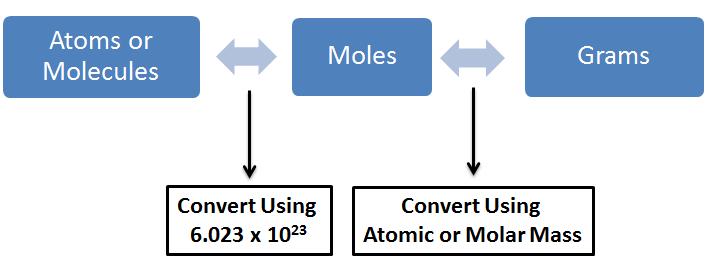
You can never relate the mass of a substance to the atoms of that substance. The number of moles must always bridge the gap between the two units.
I must also add that
With that let's begin:

