How many stereoisomers of 2-chloro-3-methylbutane exist?
1 Answer
Dec 3, 2015
Four.
Simply put, there are two configurations per stereocenter on a compound with all
Also, although meso isomers could reduce the number of stereoisomers, there are none here because clearly,
As a result, there are
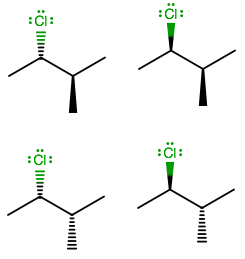
And if you worked it out, you can see that you only have permutations of (R, R), (R, S), (S, R), and (S, S).

