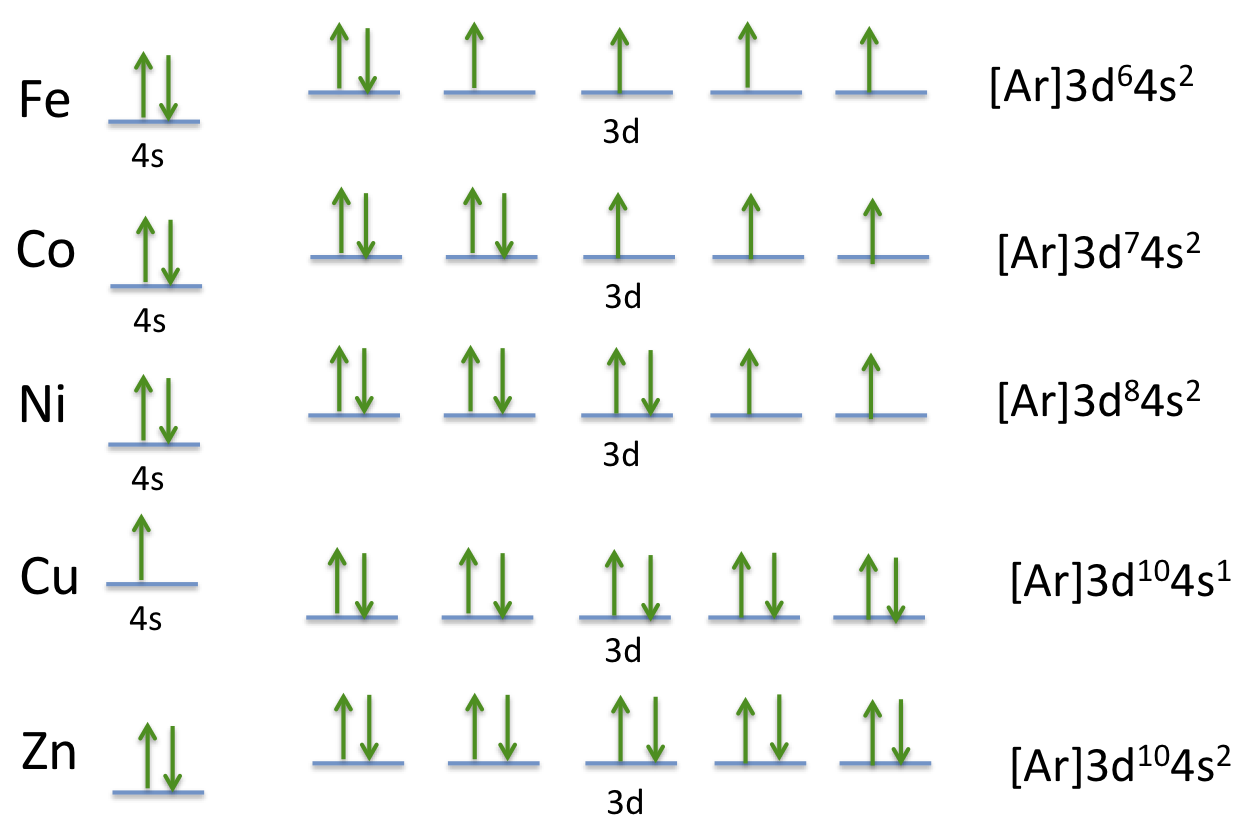
How many unpaired electrons are in an atom of Co in its ground state ? A) 7 B) 1 C) 3 D) 2
1 Answer
The answer is C) 3.
The ground state electron configuration is
However, when adding electrons to the orbitals within a sublevel, one electron will be added to each orbital, and each with the same spin (Hund's rule), after which additional electrons will be added with a maximum of two, each having an opposite spin. (Pauli exclusion principle).
So if we look at the ground state electron configuration of cobalt, we see that the