How This two reducing agent act (LiAlH4 and NaBH4 ) on breaking different type of bond like (c=c,c=o e.t.c) in different compound?
Please describe it clearly.It confuses me alot... Please provide side to side difference if possible...
Please describe it clearly.It confuses me alot... Please provide side to side difference if possible...
2 Answers
Both
Explanation:
And thus we get....
...the aluminium reagent has THREE more hydrides to transfer....
Reduction of an olefin, i.e.
On the other hand, sodium borohydride is a bit less potent as a reductant...and a bit less reactive....
Here's what I get.
Explanation:
Both reagents normally reduce
For example,
The only alkene double bonds that either reagent reduces are those in
α,β-unsaturated carbonyl compounds.
A. Lithium aluminium hydride (LAH)
LAH reduces alkene double bonds only to a minor extent.
However, it reduces propargylic alcohols to alcohol to (
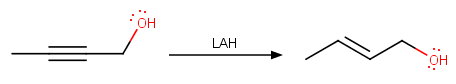 propargyl
propargyl
B. Sodium borohydride
For example, the reduction of cyclohex-2-enone gives an almost 50 % yield of cyclohexanol.
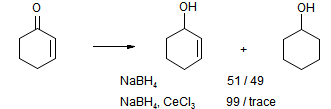 Unsat
Unsat
However, reduction in the presence of


