How to arrange ions in order of increasing ionic radius?
I have to arrange Al^(3-),Se^(2+),K^+,and Br^+ in order of increasing ionic radius.
I'v tried, but don't know if I am correct. My answer
is Se^(2+) < Br^+ < Al^(3-) < K^+
Thanks in advance.
I have to arrange
I'v tried, but don't know if I am correct. My answer
is
Thanks in advance.
1 Answer
Do you agree that while CATIONS should be smaller than their parent atoms....
Explanation:
....ANIONS should be LARGER than their parent atoms?
Well, why?
Reasonably, we address the radii of ions and atoms, by the radius of their VALENCE electron. And of course with respect to a row, a Period of the Periodic Table as we face the Table, the size atoms (defined by their valence electronic radius) should DECREASE, and that with respect to a column, a Group of the Periodic Table, atomic radii should INCREASE down the Group. And this is basic and assumed knowledge for 1st year.
And so we repair to the Table....and we compare, 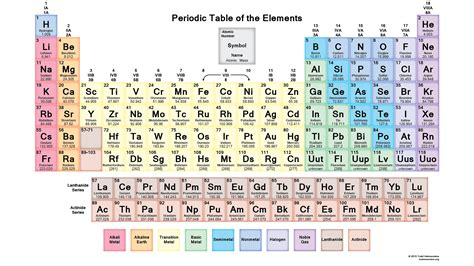 wikimedia.org
wikimedia.org
Aluminum is a third-row element...and thus it SHOULD be smaller
than 4th row potassium, selenium, and bromine...because its valence shell is
And now to the anions, and cations....and for a given PERIOD, a CATION should be smaller than an anion, inasmuch as we remove an electron, and the remaining electrons should be (and are) held more tightly at closer radii due to the absence of electronic shielding.
And finally, you are a scientist, and you should have recourse to quantitative measurement: find atomic radii on the web for elements, and cations, and anions, and see whether or not the given argument is kosher!

