what is the difference between the reactivity and abundance of elements in Group 3A through Group 8A?
1 Answer

List down the elements in Group 8A
compounds.
Well I've listed the abundance of each element in Group 8A
Argon = 0.96%(in the atmosphere) +
Helium =
Neon =
Krypton =
Xenon =

Source :Crust: CRC Handbook
List down the elements in Group 3A
Boron =
Aluminium =
There's also 2.2% aluminium in the mantle
Gallium =
Indium =
Thallium =
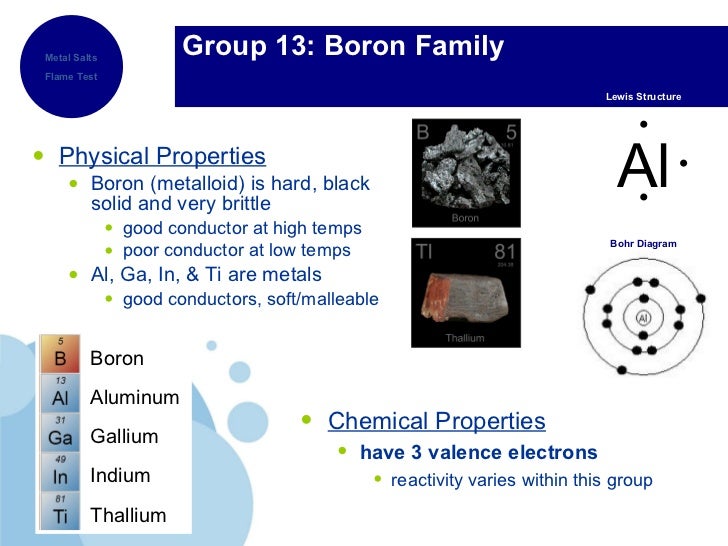
I've listed the abundance of each element in Group 3A
So we can see that the abundance of the elements in Group 3A is more than that of abundance of elements in Group 8A
And since noble gases are non-reactive the elements in Group 3A are more reactive than the elements in Group 8A

