How to count formal charge in NO2?
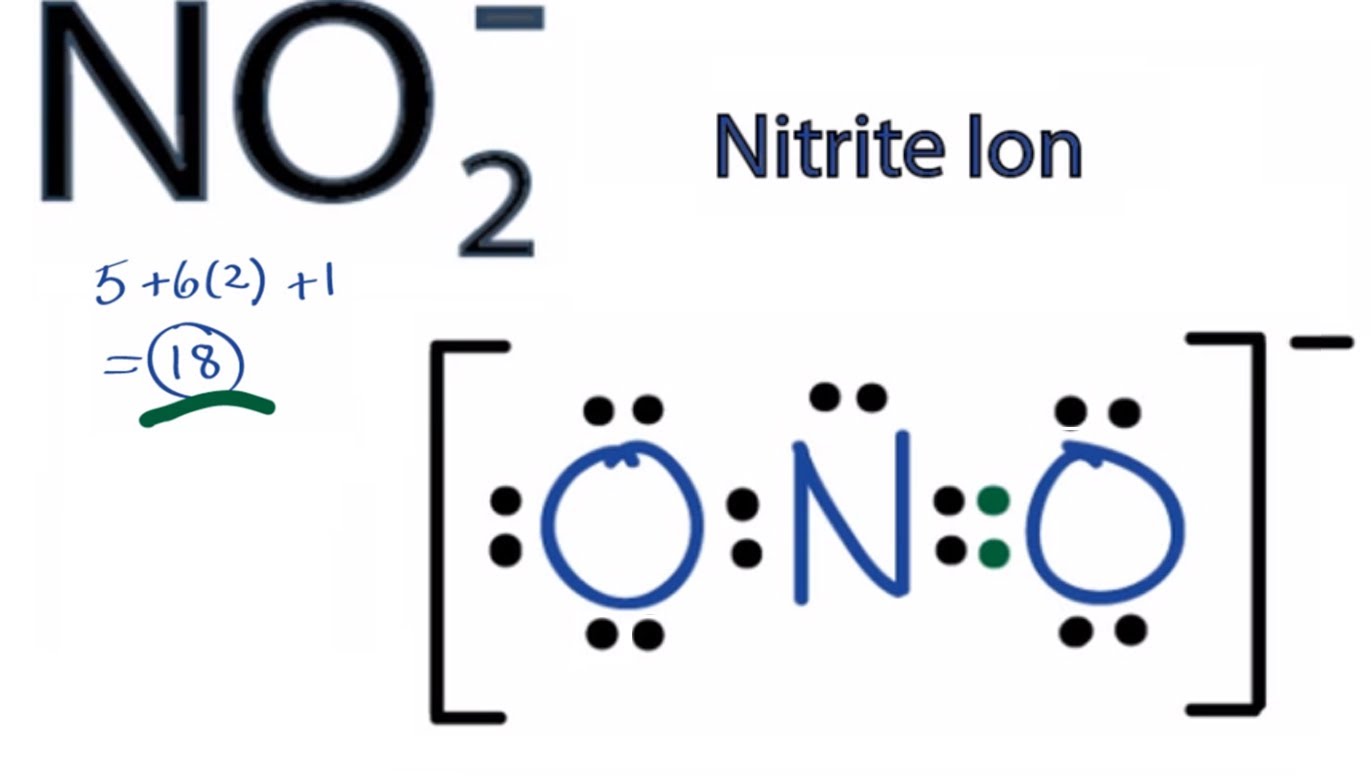
How to count formal charge in Nitrite Ion?

How to count formal charge in Nitrite Ion?
1 Answer
Nov 29, 2017
In nitrite ion? It looks like you done it mate.
Explanation:
We gots
And so
Of course we can distribute the negative charge over the two oxygen centres by resonance, and so

