How to decide which hydrogen would be the leaving group in compounds such as bicyclo[3.3.1]nonane?More specifically why does the bromine substitution occur at the hydrogen attached to the bridgehead and not the other hydrogens attached to the carbons .
Also calculate the charges of each atom
Also calculate the charges of each atom
1 Answer
At least in the first mechanistic step, everything is neutral... it's a radical reaction, i.e. homolytic cleavage occurs (or should occur) all the way through.
Okay, so you're talking about the product:
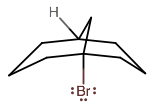
and why the hydrogen radical,
If you notice the number of bonding groups around the bridgehead carbons, there are four:
- one from each side
- one from the bridge
- one from the
#"H"# (or in the product, a#"Br"# )
That generates a tertiary carboradical if the
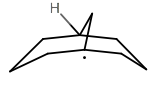
Carboradicals follow the same thermodynamic stabilization trend as carbocations. They are roughly planar.
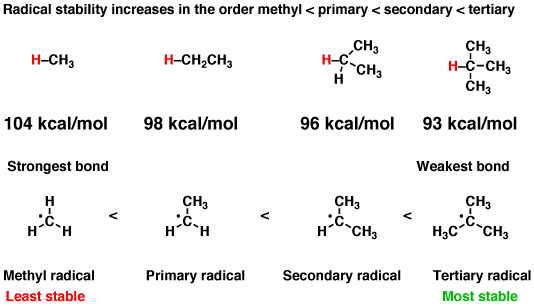
(Granted, the differences in stability are subtle. Being within
#"1 kcal/mol"# is considered chemical accuracy.)
This is because hyperconjugation from an adjacent
And since the compound was originally symmetrical (

