How to draw resonating structures of nitrogen oxides. Need their average/hybrid structures too...like we draw a circle in hexagon while representing Benzene?
1 Answer
May 28, 2018
Here's ONE example:
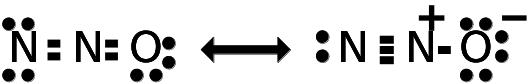
Explanation:
Nitrous Oxide structure is linear (8 electron pairs, 4 bonding pairs and 4 lone pairs)
N=N=O <-> N=N-O
Nitrous oxide exists as a resonance hybrid of the following resonance structures:
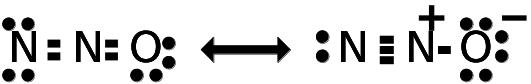
The above Lewis structure shows “no” lone pairs on central atom
Bond pairs = 2 [2 sigma bonds arranged at 1800 angle, giving Linear shape/geometry to the molecule]
Writing them out usually is just using the equilibrium arrows between the different structures to indicate equal movement between them.. They do not all have a particular 'short hand' notation like that used for delocalized bonds in hydrocarbon rings.

