How to draw the potential energy diagram for this reaction?
2 Answers
Since heat is released for
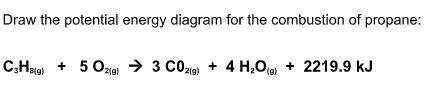
#"C"_3"H"_8(g) + 5"O"_2(g) -> 3"CO"_2(g) + 4"H"_2"O"(g) + 2219.9" kJ"# ,
we say that
The above is for an endothermic reaction.
A certain feature of combustion reactions suggests that you should draw the diagram DIFFERENTLY from what you see above. How should the energy of the products compare with that of the reactants for combustion reactions?

Explanation:
1. Identify the general shape of the energy diagram
Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process.
The enthalpy of a system,
The vertical axis of the graph resembles chemical potential energies; as previously stated, the reactants have chemical potential energies higher than that of the products and should thus be placed higher than the products.
The activated complex- as resembled by the peak between the reactants and the products- however, possesses the most significant amount of chemical potential energy among all three states since by definition the complex (intermediate state) can spontaneously proceed either forward to the products or backward to the products without any additional inputs of energy.
2. Identify the sign of the enthalpy change,
The decrease in chemical potential energy should be the same as the amount of thermal energy released. That is
However, since
Note that by convention, the enthalpy change is typically represented with an arrow pointing from the energy level of the reactants to that of the products. This fact also agrees with the conclusion of the sign of


