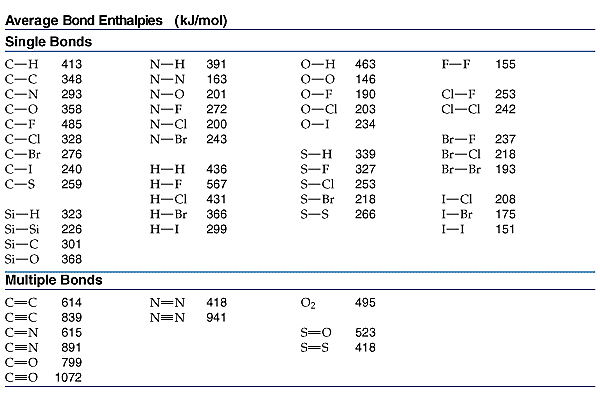
How to estimate (Delta H) for the bromination of ethylene from bond dissociation energies ?
1 Answer
Apr 5, 2015
This problem is an application of Hess's Law.
We break all the bonds to form atoms, and then we reassemble the atoms to form new bonds.
The equation is
H₂C=CH₂ + Br-Br → Br-CH₂-CH₂-Br
We can ignore the C-H bonds, because they are just being broken and re-formed.
We need a table of bond energies like the one below.