How to find how many moles are in an ion?
I am given the solution of NaCl and it has a mass of 53.2 g and a molar mass of 58.44 g. I already know to how to find the number of moles for the entire solution, but I also need to find the number of moles for Na + ions. How exactly to I go about doing that? Thanks in advance!
I am given the solution of NaCl and it has a mass of 53.2 g and a molar mass of 58.44 g. I already know to how to find the number of moles for the entire solution, but I also need to find the number of moles for Na + ions. How exactly to I go about doing that? Thanks in advance!
1 Answer
Explanation:
You're dealing with a soluble ionic compound, so you know for a fact that it dissociates completely in aqueous solution to produce cations, which are positively charged ions, and anions, which are negatively charged ions.
In this case, you know that one formula unit of sodium chloride,
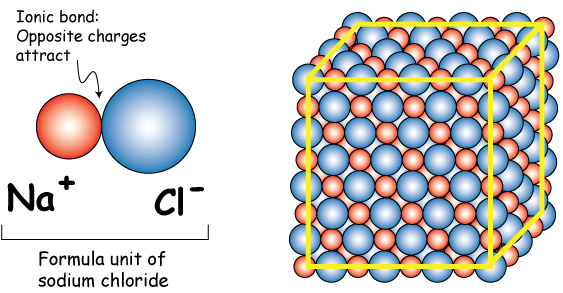
This means that when you dissolve one mole of sodium chloride in water, you will get one mole of aqueous sodium cations and one mole of aqueous chloride anions.
#"NaCl"_text((aq]) -> "Na"_text((aq])^(+) + "Cl"_text((aq])^(-)#
So, if every mole of sodium chloride produces One mole of sodium cations, it follows that the number of moles of sodium cation present in your solution will be equal to the number of moles of sodium chloride you dissolved to create this solution.
More specifically, you know that you have
#53.2 color(red)(cancel(color(black)("g"))) * overbrace("1 mole NaCl"/(58.44color(red)(cancel(color(black)("g")))))^(color(purple)("molar mass of NaCl")) = "0.910 moles NaCl"#
This corresponds to
#0.9103color(red)(cancel(color(black)("moles NaCl"))) * "1 mole Na"^(+)/(1color(red)(cancel(color(black)("mole NaCl")))) = color(green)(|bar(ul(color(white)(a/a)"0.910 moles Na"^(+)color(white)(a/a)|)))#
The answer must be rounded to three sig figs, the number of sig figs you have for the mass of sodium chloride.

