How to make these molecules from cyclopentene?
Starting from cyclopentene how would you prepare these molecules:
a) chlorocyclopentane
b) 3-bromocyclopentane
c) cyclopentanol
d) cyclopentylcyclopentan
Starting from cyclopentene how would you prepare these molecules:
a) chlorocyclopentane
b) 3-bromocyclopentane
c) cyclopentanol
d) cyclopentylcyclopentan
1 Answer
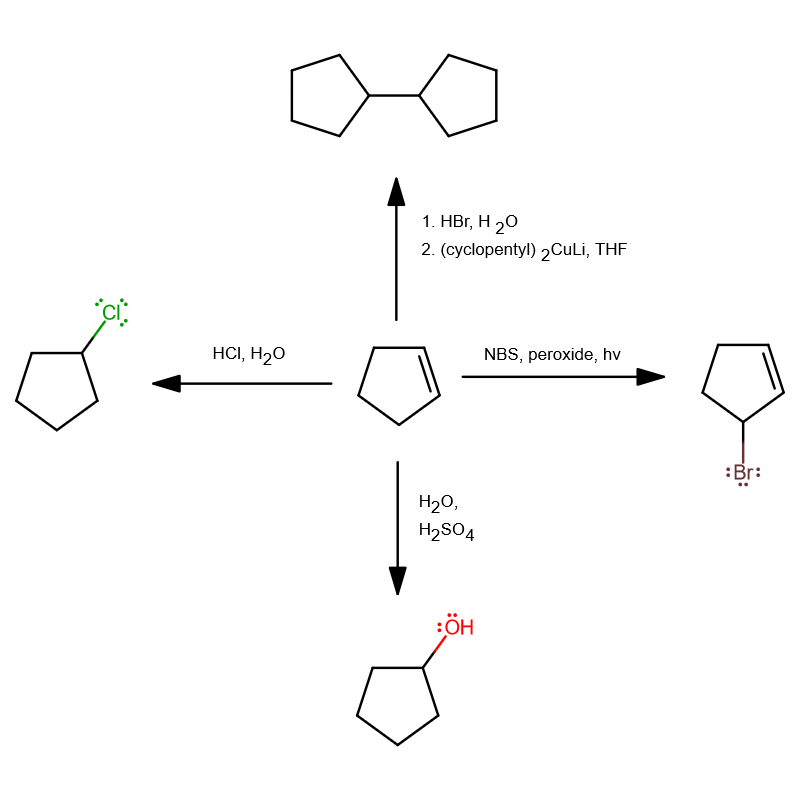
Chlorocyclopentane will have no specific designation as to which carbon is carbon-1, so it does not matter which atom in the double bond is chlorinated. Aqueous
#"HCl"# would do the trick.
3-bromocyclopentane is the same as bromocyclopentane. I think you may mean 3-bromocyclopentene, where the 3 has a meaning.
To do this, using
#N# -bromosuccinimide with a peroxide and light or heat puts a bromine atom on the allylic carbon (one atom away from an alkene carbon).Since cyclopentene has a reflection plane bisecting its double bond, the regular product and the resonance product (where the double bond conjugates down to be across carbon-3 and carbon-2) are identical.
If you mean the alkane, just finish by subsequently adding
#H_2# catalyzed by#Pd# over#C# .
Similar to
#(a)# , it does not matter which alkene carbon gets the#"OH"# , so simple acid-catalyzed hydration---which is Marknovnikov---works just fine (rather than, say, hydroboration-oxidation, which is anti-Markovnikov), i.e.#"H"_2"O"# and#"H"_2"SO"_4# .It would work better if the
#"H"_2"SO"_4# were dilute.
This one's kinda tricky, and requires you to look for a specific reaction to do in one step.
You can do this with a Gilman reagent, which is most useful for coupling two alkyl groups starting from an alkyl halide and an alkyllithium reagent.
If you prepare your Gilman reagent:
#2"cyclopentylLi" + "CuI" stackrel("THF"" ")(->) ("cyclopentyl")_2"CuLi" + "LiI"# then you can use it with the newly-formed bromocyclopentane dissolved in
#"THF"# to dimerize and form your cyclopentylcyclopentane.

