How to memorize and understand the mechanism in preparation and reactions of alkene?
2 Answers
Chemistry is not intended to be "memorized".
Explanation:
Of course explaining a question like this here would take hours of writing but I'll try to hint you on how to approach studying this topic.
First of all you must understand that alkene chemistry relies greatly on the fact that they have a weak pi bond. Since this bond is chemically "fragile", it would be easily broken in reactions and the agent that broke it would bond to the atoms that had the double bond.
Ok, how would this bond be broken?
If you may realize, the pi bond is like an electron cyclone. Electrons are just going crazy in large orbits (that is why it is fragile). You would now think that an "electrophile" would tend to react with an alkene. An electrophile is basically a molecule that craves electrons. It would catch those lose pi electrons and easily break the double bond.
If you understand what I wrote above then congrats! You've just cracked the code for most of alkene reactions.
Now synthesis of alkenes is just the opposite. You have to remove two atoms ore groups attached to two adjacent carbons. The electrons on the carbons would find nowhere to go so they'd just form a double bond.
Could any two atoms or group just leave? No.
They must be good leaving groups (i.e. groups that could exist in the reaction medium in a stable manner independently.). If the groups on two adjacent carbons are not good leaving groups then you must add something to the reaction to convert them into such.
I hope this helps.
I personally do not memorize the reactions or mechanisms, but instead, I practice and it eventually becomes easier to just figure out what happens. I learn the behavior of electrons within different environments.
For example, consider the basic reaction of ethene with
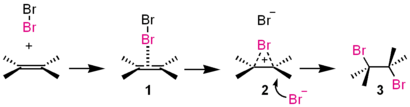
The double bond on ethene is very electron-rich. That tends to make something a nucleophile, knowing that a nucleophile is an electron donor. Naturally, an electrophile, an electron-lover, wants those electrons.
Seeing how ethene becomes a nucleophile, one of the
The pi bond on ethene can then donate two electrons to the closest
Number of arrows: 3
Now that you have this positively-charged, sterically-unstable intermediate (2), the
Number of arrows: 2
And that's it, the reaction is over. You get 3, an anti-addition of two

