How to prepare 1-phenyl-3-methyl -2- butanol?? Thank you in advance
1 Answer
Sep 3, 2015
If we were to do a "practical" approach (where you start from an easy-to-remember compound), starting from benzene isn't too bad of an idea.
I'll use the line structure notation since I hope you are accustomed to that at the time of writing your thesis. ;)
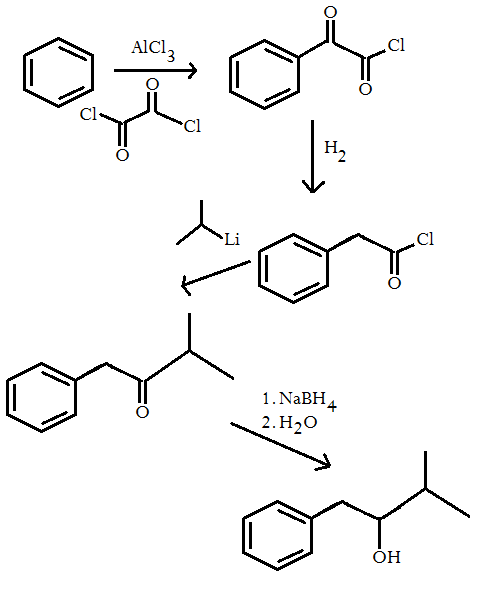
The steps I used are:
- Friedel-Crafts Acylation using oxalyl chloride (yes, I was allowed to use this on an exam)
- Reduction of the carbonyl adjacent to the benzene ring (does not affect other carbonyls not adjacent to the benzene ring)
- Organolithium reagent as a nucleophile to attack the carbonyl, with a chloride leaving group
- Sodium borohydride reduction to a secondary alcohol
- Water to end the reaction in step 4 by protonating the
#O^(-)# atom

