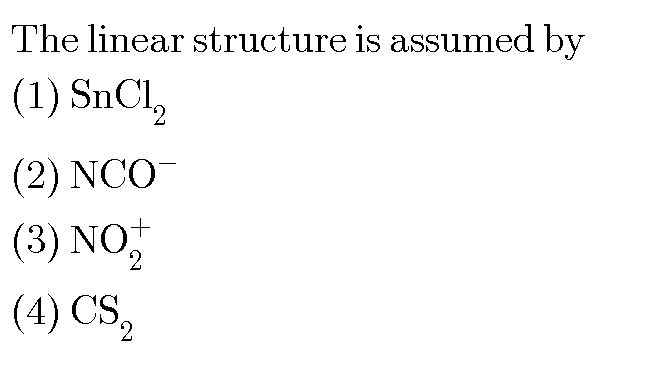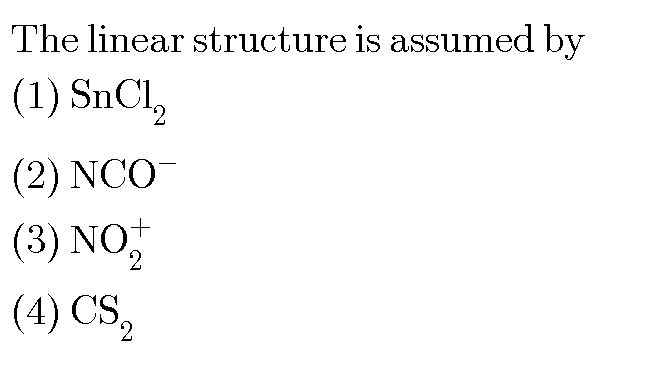We simply apply VESPER....
#SnCl_2#...there are 3 electron pairs around the tin centre....#Cl-ddotSn-Cl#, and thus #/_Cl-Sn-Cl~=120^@#...#"stannic chloride"#, #SnCl_4# would be tetrahedral, why?
#:N-=C-O^(-)#, we gots #5+4+6+1=8# #"electron pairs"# to distribute.... and linear geometry with respect to #/_N-C-O=180^@#..of course the alternative Lewis structure...#""^(-):stackrel(ddot)N=C=O^#, is ALSO linear.
#O=stackrel(+)N=O^#, we gots #6+5+6-1=8# #"electron pairs"# to distribute.... and linear geometry with respect to #/_O-N-O=180^@#..we conceive that the formal charge lies on nitrogen.
#S=C=S#, we gots #6+4+6=8# #"electron pairs"# to distribute.... and linear geometry with respect to #/_S-C-S=180^@#..if you have ever used this stuff you would remember its pen and ink, and also it is VERY inflammable.