How to write the name of Hau3+ ion using stock notation. For example HAuCl4 is written as.......(lll) chroride. Fill the blank space?
1 Answer
Sep 28, 2017
See explanation
Explanation:
To explain this better I'll use reversible reactions.
You think that:
Instead, it is:
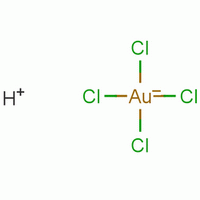
So, there is no

By the way, the only compound I know fitting the name of _(III) chloride, is Gold(III) chloride (

