How todraw all stereoisomers of 2-bromo-4-methylpentane?
2 Answers
Make a model....
Explanation:
You gots...
AS written
And once you have drawn one geometry, the interchange of ANY 2 substituents around
Is
Here's how to do it.
Explanation:
The structure of 2-bromo-4-methylpentane is
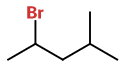 2Br4Me
2Br4Me
There is only one chiral centre,
Draw a wedge-dash structure. Put the
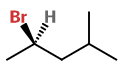 R
R
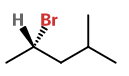 S
S


