How would the molecule below be named based on IUPAC naming?
1 Answer
The IUPAC name is (
Explanation:
Step 1. Start with the ring system
The carbonyl carbon is
Then you number the ring carbons counterclockwise, giving the double-bonded carbons priority in numbering.
The base name is cyclohex-2-en-1-one
Step 2. Name the substituents
We have a methyl group at
The name is now 2-methyl-5-(prop-1-en-2-yl)cyclohex-2-en-1-one
Step 3. Assign stereochemistry
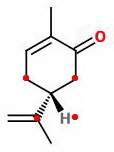
All we can say is that the
We must go one atom further out to break the tie.
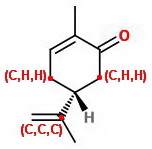
The double bond counts as two
This breaks a tie. The propenyl carbon is priority 1.
We must go still further out from
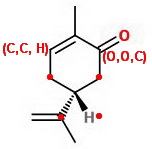
This breaks the tie! (
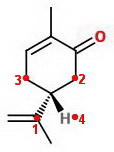
The 1→2 →3 direction is counterclockwise (
However, the
The name of the compound is
(
Its common name is (-)-carvone.

