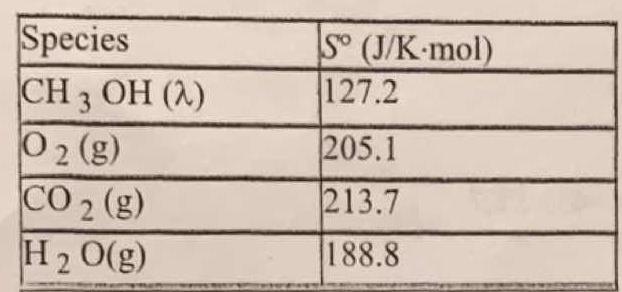
How would you calculate the standard entropy change for the combustion of methanol at 25 C? #2CH_3OH(lamda) + 3O_2(g) -> 2CO_2(g) + 4H_2O(g)#
1 Answer
Apr 18, 2017
You would calculate it like this:

