How would you explain the ionization energy trend?
1 Answer
On the basis of
Explanation:
For ionization energy we measure the energy with the transition....
That is the energy with the formation of one mole of GASEOUS CATIONS, and one mole of GASEOUS ELECTRONS, FROM ONE MOLE of GASEOUS ATOMS....
Two factors influence this ionization energy: (i) the nuclear charge, i.e.
And these trends are reasonable on the basis of simple ideas regarding electrostatics...the valence, the outermost electron, is FARTHER REMOVED FROM the nuclear core, and thus it is MOST LOOSELY held by the parent atom.
And as chemists, as physical scientists, we should look to the data... See...
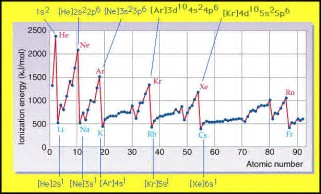
Are these data consistent with the argument? Why or why not?

