How would you find the molecular formula for a compound with the empirical formula CH2O and the molar mass is 180.2 g/mol?
1 Answer
Explanation:
A compound's empirical formula tells you the smallest whole number ratio between the elements that make up said compound is.
This means that you can think about the empirical formula as being a sort of building block for the molecule.
Looking at the empirical formula for your compound,
- one carbon atom
- two hydrogen atoms
- one oxygen atom
in order to start building this molecule. Your job now is to determine how many of these building blocks are needed to get the molecular formula of the compound.
Notice that you know the molar mass of the compound. The molar mass tells you what the total mass of one mole of the compound is.
This means that if you figure out the molar mass of a building block, you can use the total molar mass of the molecule to determine how many buildings blocks you need.
To get the molar mass of the empirical formula, use the molar masses of each atom it contains
overbrace(1 xx "12.011 g/mol")^(color(blue)("one mole of C")) + overbrace(2 xx "1.00794 g/mol")^(color(green)("two moles of H")) + overbrace(1 xx "15.9994 g/mol")^(color(red)("one mole of O")) = "30.03 g/mol"
So, one building block has a molar mass of
30.03 color(red)(cancel(color(black)("g/mol"))) * color(blue)(n) = 180.2 color(red)(cancel(color(black)("g/mol")))
color(blue)(n) = 180.2/30.03 = 6.0007 ~~ 6
The molecular formula of the compound will thus be
("CH"_2"O")_color(blue)(6) implies "C"_6"H"_12"O"_6 -> glucose
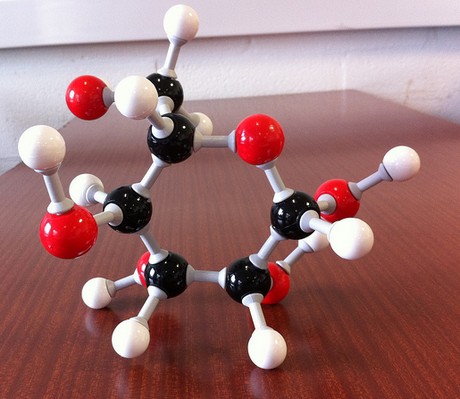 http://www.nutritionalhq.com/about-carbohydrates/about-glucose/
http://www.nutritionalhq.com/about-carbohydrates/about-glucose/

